
-
About US
About US
- Patients
-
Professional Medical Solutions
Professional Medical Solutions
- News
- Investor Relations

AcoArt Tulip® & Litos®
Below-the-knee DCBBelow-the-knee DCB


· Lipophilic excipient matrix + paclitaxel, improving drug transfer rate and optimizing drug conveying

· Provide various product specifications from 20 to 300mm, satisfying the needs for treating different complex lower limb lesion. A minimum diameter of 2mm is available for accessing toes
Chinese clinical trial: Enroll patient baseline
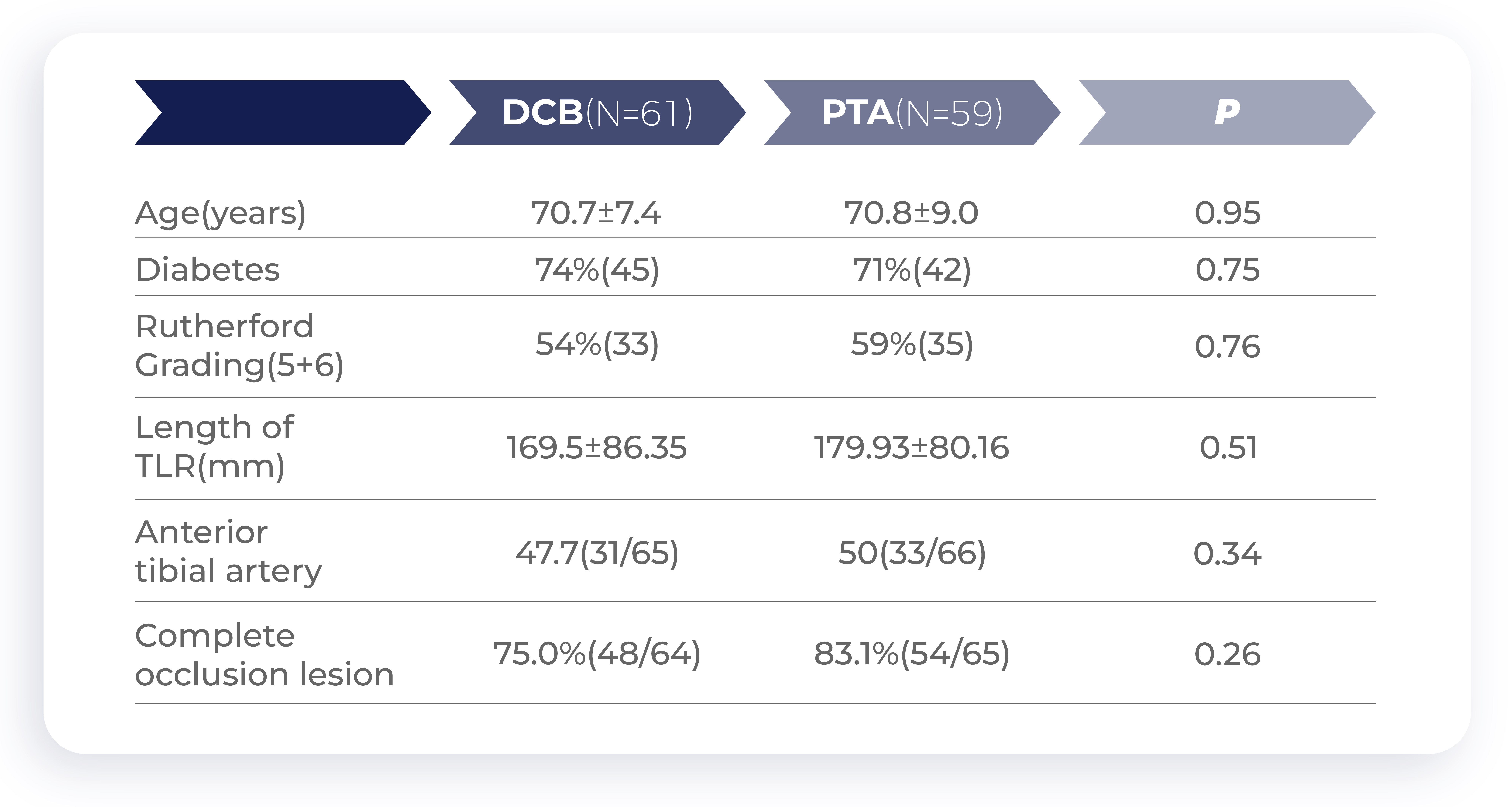
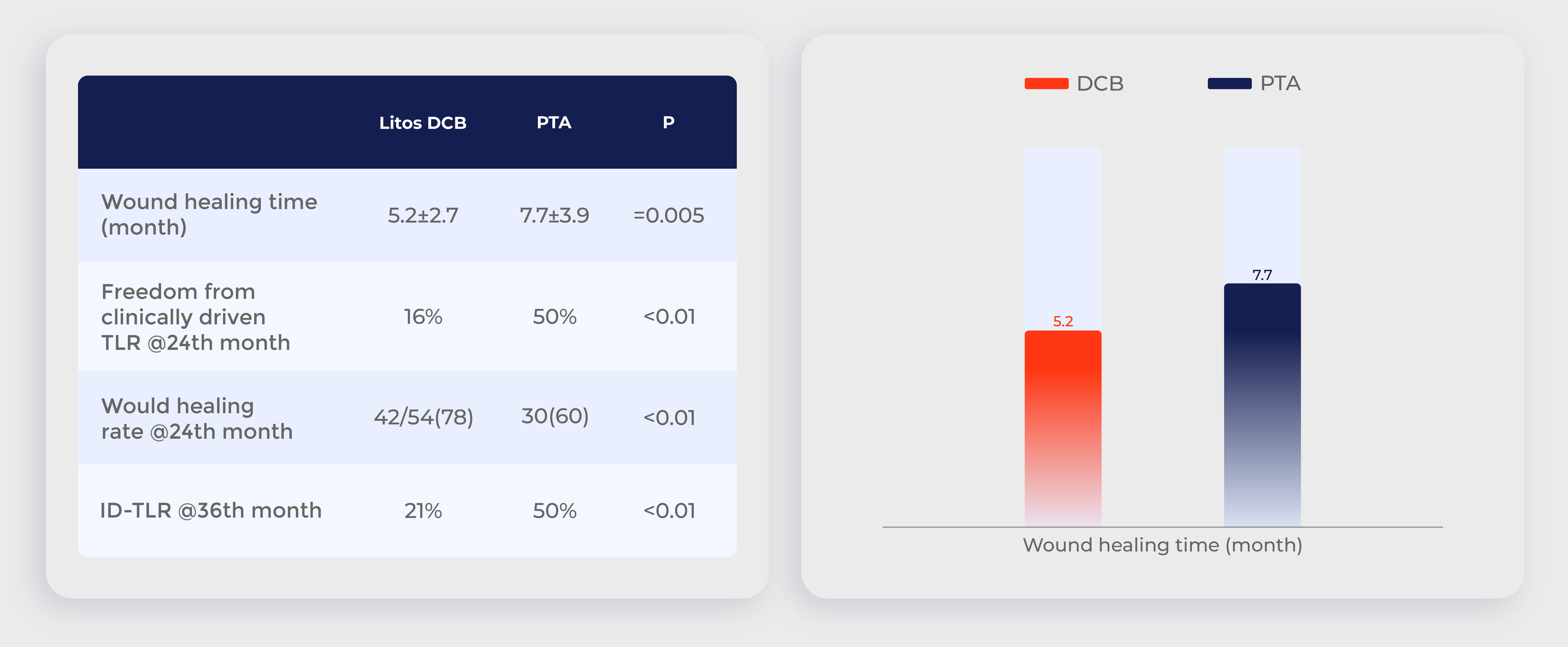
Chinese clinical trial: DCB clinical efficacy is significantly higher than that of PTA
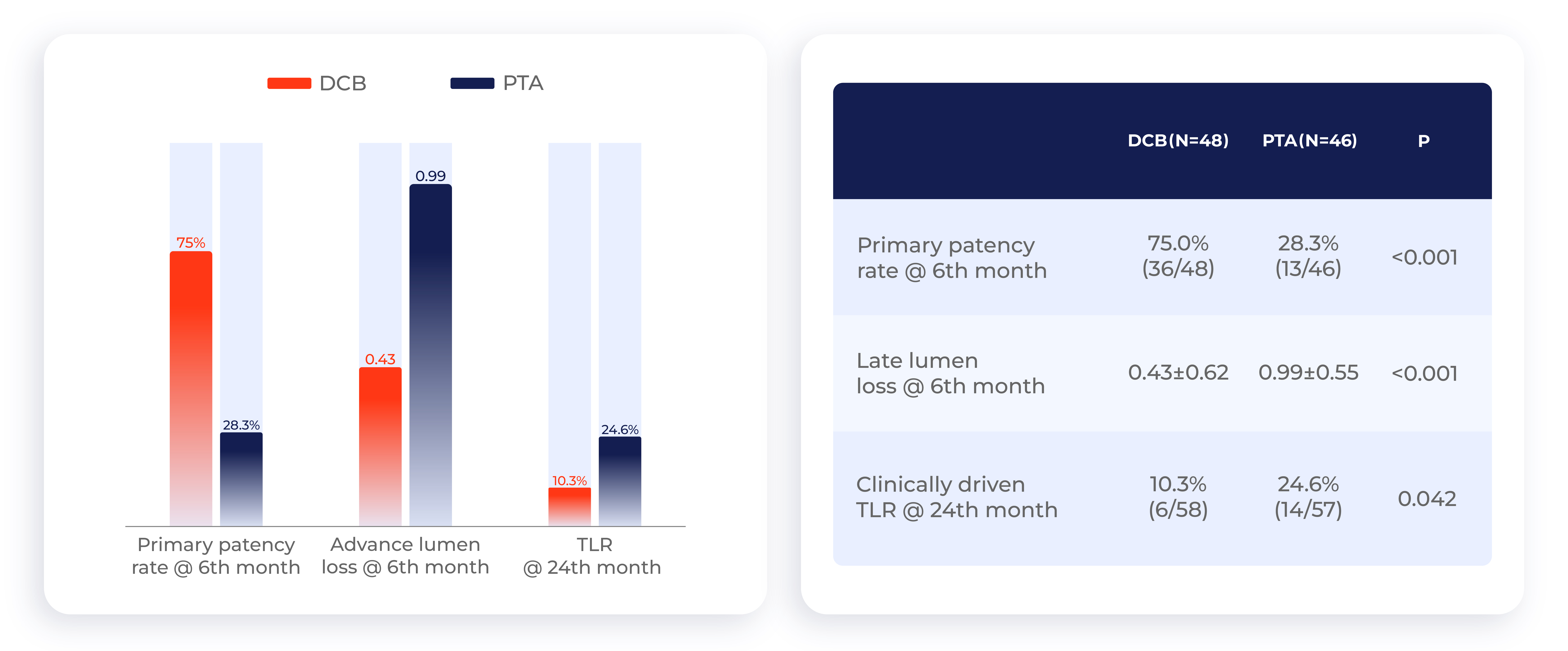
Chinese clinical trial: 5-year data show that the long-term patient survival rate of DCB is higher than that of PTA
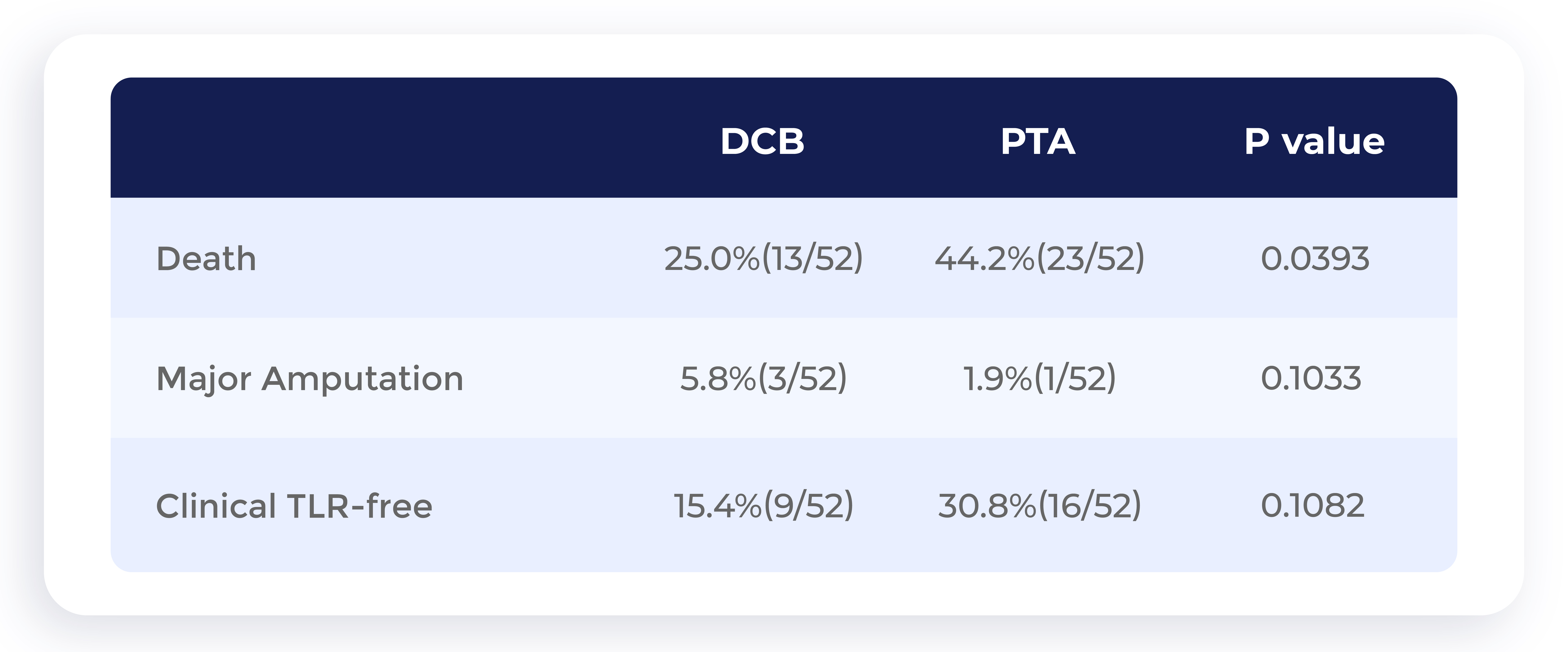
Data source:Drug-Coated Balloon Angioplasty Compared With Uncoated Balloons in the Treatment of Infrapopliteal Artery Lesions (AcoArt II–BTK),J Endovasc Ther. 2021 Apr;28(2):215-221.
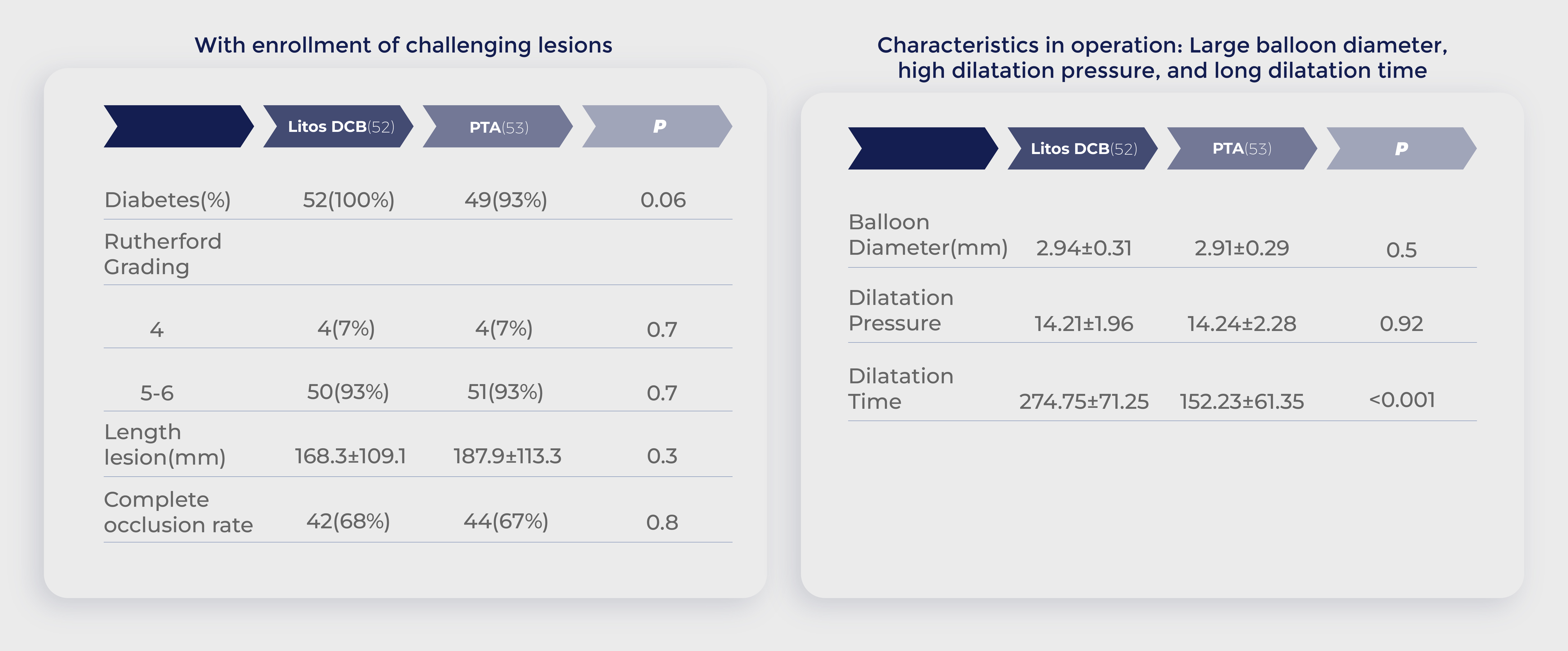
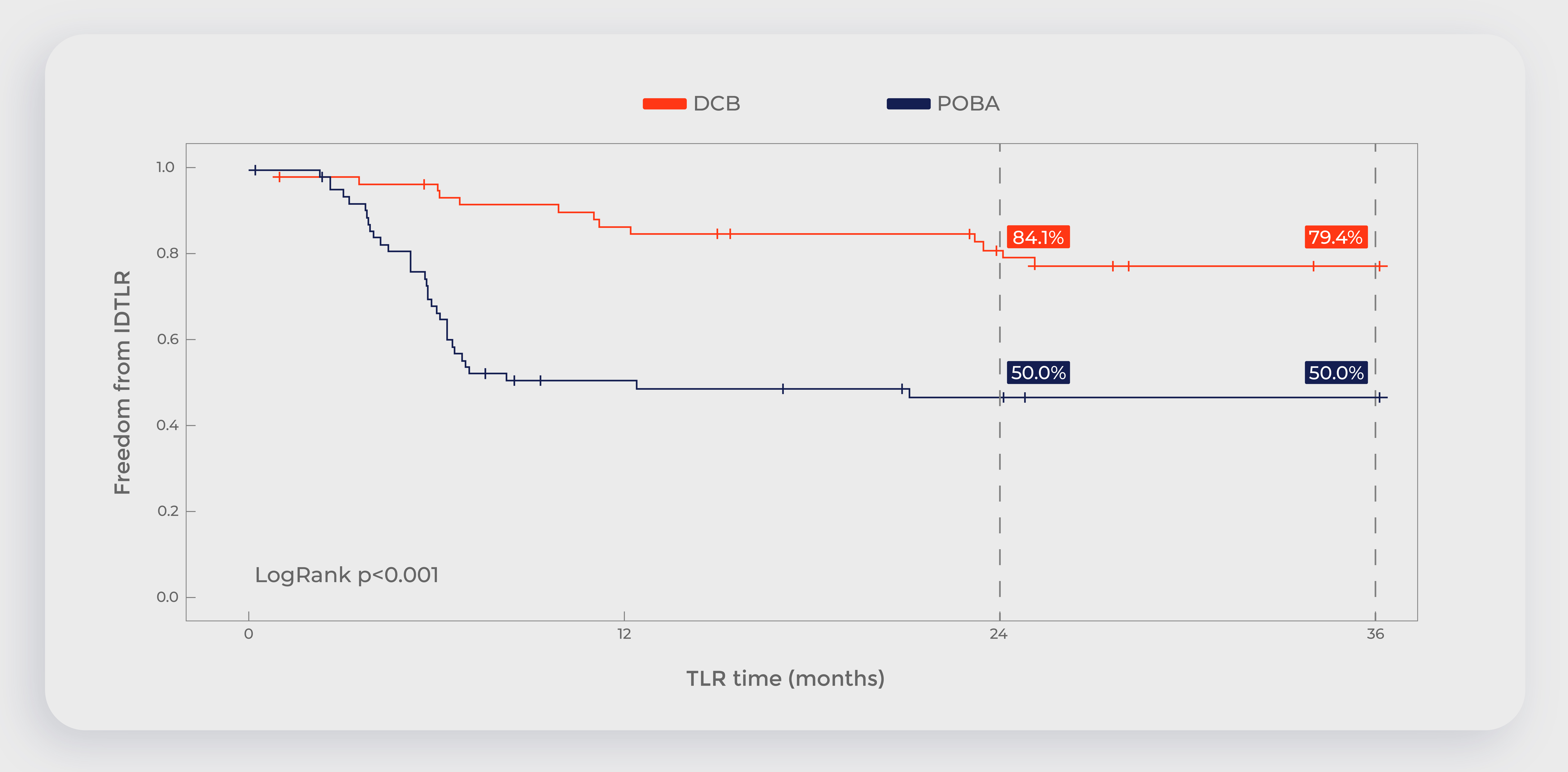
Data source:Randomized Controlled Trial of Acotec Drug-Eluting Balloon Versus Plain Balloon for Below-the-Knee Angioplasty,JACC Cardiovasc Interv. 2020 Oct 12;13(19):2277-2286.
| Name | AcoArt Tulip® | AcoArt Litos® |
Drug | Paclitaxel | Paclitaxel |
Excipient | Magnesium stearate (lipophilic) | Magnesium stearate (lipophilic) |
Diameter(mm) | 2/2.5/3/3.5/4 | 2/2.5/3/3.5/4 |
Length(mm) | 20/30/40/60/80/100/120/150/200/250/300 | 20/30/40/60/80/100/120/150/200/250/300 |
Guidewire(inch) | 0.018 | 0.014 |
Sheath(F) | 4-5 | 4 |
NP(Bar) | 6 | 6 |
RBP(Bar) | Max.18 | Max.18 |

Copyright © 2023 先瑞达医疗科技控股有限公司 京ICP备19054161号-2 MINETHINK